Isotherm of Al-Mg-Zn at 500°C
Purpose: Learn to calculate and use an isothermal section in a ternary system
Module: PanPhaseDiagram
Thermodynamic Database: AlMgZn.tdb
Batch file: Example_#1.5.pbfx
A complete phase diagram of a ternary system should be described by a triangular prism in a 3D space with each edge of the triangle base representing the composition axis of each component and the vertical axis the temperature. Due to the complexity of many ternary systems, phase stability of a ternary system is usually described by Isothermal sections parallel to the triangle base (constant temperature) and isoplethal sections vertical to the triangle base plane. In this example, we will calculate an isothermal section of Al-Mg-Zn at 500°C.
Calculation Procedures:
-
Load AlMgZn.tdb following the procedure in Pandat User's Guide: Load Database , and select all three components;
-
Perform 2D calculation following the procedure in Pandat User's Guide: Section Calculation (2D) ;
-
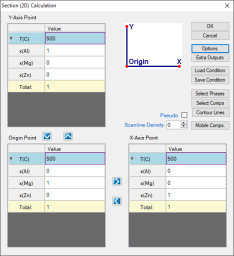
Set Calculation Condition as shown in Figure 1;
Post Calculation Operation:
-
Label phase field following the procedure in Pandat User's Guide: Icons for Graph on Toolbar;
-
Change graph appearance following the procedure in Pandat User's Guide: Property;
-
Add tie-lines following the procedure in Pandat User's Guide: Property;
Information obtained from this calculation:
-
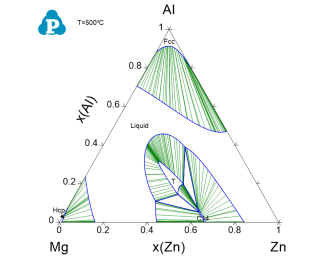
Phase stability in different composition areas, single phase, two-phase, and three-phase regions, can be viewed clearly at this temperature;
-
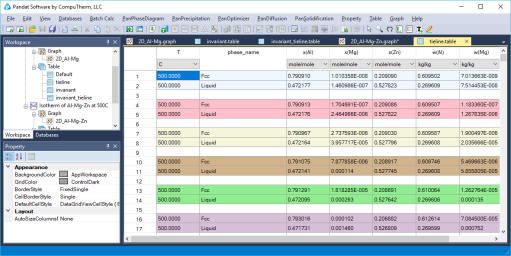
Equilibrium between two phases is connected by tie-lines, and the equilibrium compositions of the two phases can be read at the end points of the tie-line; Details on the tie-lines can be found in Table->tieline as shown in Figure 3. In this table, the compositions of the two phases connected by each tieline are listed for all the calculated tielines;
-
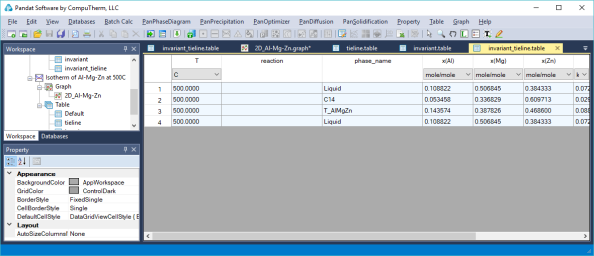
Three-phase equilibrium is shown by the tie-triangle. The composition of each phase in the three-phase equilibrium is listed in invariant_tieline table, as shown in Figure 4.